
Water
Quality Assessment: Chemical: Alkalinity
The natural buffering
capacity of a stream may mask the presence of acidic or basic
pollutants. Therefore simply measuring a stream's pH
may not be sufficient. For a more complete assessment of water quality,
most scientists also measure streamwater alkalinity.
Alkalinity is measured to determine the ability of a stream to resist
changes in pH. That is to say alkalinity allows scientists to determine
a stream's buffering capacity.
Alkalinity values of 20-200 ppm are common in freshwater ecosystems. Alkalinity levels below 10 ppm indicate poorly buffered streams. These streams are the least capable of resisting changes in pH, therefore they are most susceptible to problems which occur as a result of acidic pollutants.
The Chemistry of
Alkalinity
Alkalinity
results from the dissolution of calcium carbonate (CaCO3) from limestone
bedrock which is eroded during the natural processes of weathering. The
carbon dioxide (CO2) released from the calcium carbonate into the streamwater
undergoes several equilibrium reactions.
Equilibrium reactions are those that go in either direction, depending
on what compounds are present in the largest concentrations. The following
set of reactions is referred to as the bicarbonate buffering
system:
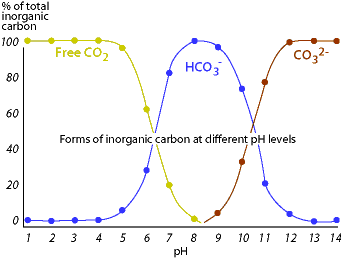
CO2 + H2O
H2CO3
H+ + HCO3-
2H+ + CO32-
As depicted in the above set of reactions, carbonate (CO32- ) and bicarbonate (HCO3-) ions act as hydrogen ion absorbers. This causes the reactions of the bicarbonate buffering system to shift left or right while maintaining a relatively constant pH. If hydrogen ions are added to the solution, they combine with available bicarbonate or carbonate ions, causing the reactions to shift to the left and eventually liberate carbon dioxide and water molecules. The addition of carbonate to the solution causes the hydrogen ions to be occupied and shifts the reactions to the right. In this way, an alkaline stream with a large buffering capacity is able to "hold" more acidic pollutant without displaying a significant decrease in pH.
Data
courtesy of Dr. Ben Stout.
Overview ..|.. Biological Assessment ..|.. Chemical Assessment ..|.. Physical Assessment.
pH
/ Alkalinity / Hardness / Nitrates.
Nitrites, and Ammonia / Ortho- and Total
Phosphate / Dissolved Oxygen and Biochemical
Oxygen Demand / Fecal Coliform / Conductivity
and Density
Glossary .|.
Related Links
.|..
References
..|..
PBL Model
.|
Home ..|.. Teacher Pages ..|.. Modules & Activities
HTML code by Chris Kreger
Maintained by ETE Team
Last updated November 10, 2004
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.
