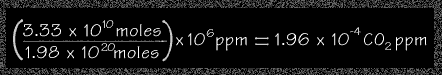Yellowstone
Biomass Burning Activity
How much CO2
was released to the atmosphere from the 1988 Yellowstone fires?
Step 1 To arrive at an approximate answer to this question, students need to determine
- Number of moles of air in the atmosphere = 3.23x1020 moles
- Moles of carbon from the
fire = 3.33 x 1010 moles of C
( see Finding Carbon Released )
Step 2 To find the approximate number of moles of air, students need to determine
- Gram molecular weight of
air in atmosphere = 29 g/mole
( see What is the gram molecular weight of the atmosphere? ) - Mass (grams) of the atmosphere
= 5.76 x 1021g
( see How to Calculate the Mass of the Air in the Atmosphere )
Using the results of Steps (1) and (2) above, students can calculate the approximate amount of CO2 ppm from the Yellowstone fires.
moles of carbon from the fire 3.33 x 1010
moles
moles of air in the atmosphere 1.98 x1020 moles
times parts per million 106 ppm
Activity
Extension
Plot a graph showing
density vs. altitude.
You may want to ask
1. How much air is within 500
meters of the earth's surface?
2. How fast does density drop?
Maintained by ETE Team
Last updated April 28, 2005
Some images © 2004 www.clipart.com
Privacy Statement and Copyright © 1997-2004 by Wheeling Jesuit University/NASA-supported Classroom of the Future. All rights reserved.
Center for Educational Technologies, Circuit Board/Apple graphic logo, and COTF Classroom of the Future logo are registered trademarks of Wheeling Jesuit University.